Abstract
The manufacture of individual patient-derived CAR T-cells is expensive, frequently unsuccessful, too time consuming to benefit acutely ill patients and difficult to scale for large numbers of patients. "Off-the-shelf" T cell products that are banked from healthy donors would improve accessibility, allow rapid treatment, and reduce costs. The major obstacles to the success of allogeneic T cells are graft-versus-host disease (GVHD) and graft rejection, mediated by host and recipient alloreactive T cells, respectively. To address GVHD, we are using allogeneic Epstein-Barr Virus-specific T cells (EBVSTs), which have not produced GVHD in more than 300 recipients. To prevent graft rejection we have expressed a chimeric antigen receptor for CD30 (CD30.CAR) in EBVSTs. CD30 will be upregulated by host alloreactive T cells when they encounter infused CD30.CAR EBVSTs. As a consequence, they will become targets for the CD30.CAR EBVSTs. Previous clinical studies (NCT02917083, NCT01555892, and NCT00062868) have shown that CD30.CAR T cells can destroy CD30+ lymphoma cells through their chimeric receptor, while EBVSTs can kill EBV+ lymphoma cells through their native TCR. Thus, once engrafted, banked CD30.CAR EBVSTs may kill both CD30+ and EBV+ lymphomas through their CAR and TCR respectively without causing GVHD.
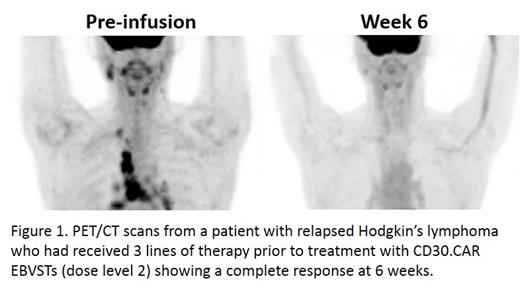
To assess the safety and activity of banked CD30.CAR EBVSTs, we treated patients with multiply relapsed (median of 4 prior lines of therapy; range 3-5) or refractory CD30-positive lymphomas in a Phase 1 dose escalation study using 4 × 10 7, 1 × 10 8 or 4 × 10 8 CD30.CAR EBVSTs infused after lymphodepletion with cyclophosphamide and fludarabine. Although CD30.CAR killing is not HLA restricted, selection of the CD30.CAR EBVST product for each recipient was based on the best HLA class I and class II match; this should allow endogenous EBV (when present) to boost the in vivo activity of CD30.CAR EBVSTs via their native TCRs, and augment reactivity in patients whose CD30+ malignancies are also EBV+. We have currently treated eight patients, including two at the highest dose level. The infusions have been well tolerated with no dose-limiting toxicities and in particular no cytokine release syndrome (CRS) or GVHD of any grade. We have evaluated seven of the patients. At 6-week evaluation, per Lugano criteria, two patients have had a complete response (one shown in Figure 1) and three have had a partial response (overall response rate of 71%). CD30.CAR EBVSTs were detectable for 1 week in peripheral blood, but there was no evidence of expansion. We are analyzing tumor samples for CD30.CAR EBVSTs, and will continue to assess the safety, efficacy and durability of these responses. Thus banked CD30.CAR EBVSTs can safely be given to allogeneic recipients and may cause significant tumor responses including complete remissions. These cells may be a suitable platform for other "off-the-shelf" CAR-T cell therapies.
Quach: Tessa Therapeutics: Research Funding. Ramos: Athenex: Research Funding; Novartis: Consultancy; Genentech: Consultancy; Tessa Therapeutics: Patents & Royalties, Research Funding. Grilley: Allovir: Current equity holder in publicly-traded company, Other: Leadership; QB Regulatory Consulting: Other: Ownership, project management support, Research Funding; Marker: Consultancy, Other: Regulatory and project management support. Brenner: Athenex: Membership on an entity's Board of Directors or advisory committees; Turnstone Biologics: Membership on an entity's Board of Directors or advisory committees; TScan Therapeutics: Membership on an entity's Board of Directors or advisory committees; Coya Therapeutics: Membership on an entity's Board of Directors or advisory committees; CellGenix GmbH: Membership on an entity's Board of Directors or advisory committees; Walking Fish Therapeutics: Membership on an entity's Board of Directors or advisory committees; Poseida Therapeutics: Membership on an entity's Board of Directors or advisory committees; Onkimmune: Membership on an entity's Board of Directors or advisory committees; Memgen: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Bellicum Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Allogene: Membership on an entity's Board of Directors or advisory committees; Abintus: Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Other: Founder; Allovir: Current equity holder in publicly-traded company; Marker Therapeutics: Current equity holder in publicly-traded company. Heslop: Kiadis: Membership on an entity's Board of Directors or advisory committees; Allovir: Current equity holder in publicly-traded company; Gilead: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Kuur Therapeutics: Research Funding; Marker Therapeutics: Current equity holder in publicly-traded company; GSK: Membership on an entity's Board of Directors or advisory committees; Fresh Wind Biotherapies: Membership on an entity's Board of Directors or advisory committees. Rouce: Tessa Therapeutics: Research Funding; Pfizer: Consultancy; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Lapteva: Tessa Therapeutics: Consultancy. Rooney: Tessa: Membership on an entity's Board of Directors or advisory committees, Research Funding; Marker Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Allovir: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal